NQuery Advisor 7.0 is usually installed in the C: Program Files (x86) nQuery Advisor 7.0 folder, regulated by the user's choice. NQuery70.exe is the nQuery Advisor 7.0's main executable file and it occupies approximately 1.05 MB (1101152 bytes) on disk. NQuery Advisor 7.0 is composed of the following executables which occupy 1.14 MB ( 1191264. The software is a useful tool for teachers, therapists, and caregivers. Use it to create support materials such as Smart Charts to help you locate vocabulary and manual boards that match the overlay in a person’s communication device, to keep everyone on the same page. ITS Software Distribution provides a single, centralized point of contact to find and obtain software on campus. Learn More If you have any questions or problems using software on this site, please visit our Online Help Desk, help.unc.edu or call 962-HELP. NQuery Advisor + nTerim. NQuery Advisor + nTerim is commercial software for calculating sample size. It is available here – student pricing is available. OpenEpi is free software for doing analysis of epidemiological data. It is available at www.openepi.com. PASS is used to find the sample size for a study. NQuery is the world’s most trusted clinical trial design platform. Covers all phases (I - IV) and all types of trial designs (classical to adaptive). Trusted by the FDA, EMA & other agencies.
| Developer(s) | Statsols |
|---|---|
| Stable release | |
| Type | |
| License | Proprietary |
| Website | www.statsols.com |
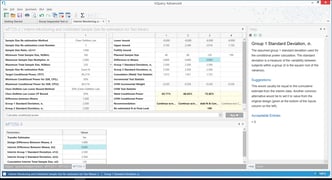
nQuery is a clinical trial design platform used for the design and monitoring of adaptive, group sequential and fixed sample size trials. It is most commonly used by Biostatisticians to calculate Sample size and Statistical power for adaptive clinical trial design. nQuery is proprietary software developed and distributed by Statsols. nQuery includes calculations for 1000+ sample size and power scenarios.
nQuery History[edit]
Janet Dixon Elashoff is a now-retired American statistician and daughter of the mathematician and statistician Wilfrid Joseph Dixon, creator of BMDP. J. Elashoff is also the retired Director of the Division of Biostatistics, Cedars-Sinai Medical Center. While at UCLA and Cedars-Sinai during the 1990s, she wrote the program nQuery Sample Size Software (then known as nQuery Advisor). This quickly became widely used to estimate the sample size requirements for pharmaceutical testing and she joined the company Statistical Solutions LLC to commercialize it.[1]
nQuery Featured In Scientific Journals[edit]
There are over 6,000 scientific studies that feature nQuery. These are available to the public for educational and research purposes.[2] The US National Institutes of Health Library lists over 895 published studies that used nQuery for sample size calculation for clinical trial design. These are freely available to the public to review.[3]

Frequentist and Bayesian statistics[edit]
nQuery allows researchers to apply both frequentist and Bayesian statistics to calculate the appropriate sample size for their study.[4]
Adaptive Clinical Trial Design[edit]
nQuery is used for Adaptive clinical trial design. Trials with an adaptive design are reported to be often more efficient, informative and ethical than trials with a traditional fixed design since they often make better use of resources such as time and money, and might require fewer participants. [5]
References[edit]
- ^Chernick, Michael R.; Friis, Robert H. (2003), Introductory Biostatistics for the Health Sciences: Modern Applications Including Bootstrap, Wiley series in probability and statistics, John Wiley & Sons, p. 360, ISBN9780471458654
- ^https://scholar.google.com/scholar?start=0&q=%22nquery%22
- ^https://www.ncbi.nlm.nih.gov/pmc/?term=nquery
- ^'What sample size and power analysis procedures you get in nQuery | Sample Size Software | Power Analysis Software'.
- ^Pallmann, Philip; Bedding, Alun W.; Choodari-Oskooei, Babak; Dimairo, Munyaradzi; Flight, Laura; Hampson, Lisa V.; Holmes, Jane; Mander, Adrian P.; Odondi, Lang'o; Sydes, Matthew R.; Villar, Sofía S.; Wason, James M. S.; Weir, Christopher J.; Wheeler, Graham M.; Yap, Christina; Jaki, Thomas (2018). 'Adaptive designs in clinical trials: Why use them, and how to run and report them'. BMC Medicine. 16 (1): 29. doi:10.1186/s12916-018-1017-7. PMC5830330. PMID29490655.

External links[edit]
Hi, I used to work in clinical research, specifically in clinical trial design and analysis. I love working things out on my own. It's often the best way to understand a phenomena. Sample size calculation is one of those finicky niche areas that require precise calculations using expensive statistical packages. I thought I would develop a free tool to estimate sample sizes, largely based on equations from the following reference:
Julious, SA. 'Tutorial in Biostatistics - Sample sizes for clinical trials with Normal data.' Statist. Med. (2004); 23:1921–1986.
I've created an Excel spreadsheet that utilizes the equations in a Microsoft Excel workbook, which arrives at the same sample sizes assoftware packages such as SAS, NQuery, and PASS. The Excel workbook is called 'Free Analysis Research Tool for Sample Size Iterative Estimation'.
To download the latest version of FARTSSIE, click the following link:
Nquery Download
Download FARTSSIE25.xlsm I now use this spreadsheet in my bioequivalence electives at the Leslie Dan Faculty of Pharmacy, University of Toronto.
I now use this spreadsheet in my bioequivalence electives at the Leslie Dan Faculty of Pharmacy, University of Toronto. Visit Dr. Russel Lenth's Website, the person who made this software possible.
software, free download Music
FEATURES:- Version 2.5: Release Date 13-Oct-20
- Removed all Reference Scaling - Redirect this method to PowerTOST
- Version 2.4: Release Date 14-Mar-19
- Corrected rendering of d on superiority and non-inferiority trials
- Version 2.3: Release Date 1-Feb-17
- Added Lehr's Formula to Superiority, Parallel worksheet
- Version 2.2: Release Date 1-Feb-17
- Added ±d values to graphs, adjusted BE limits for replicate designs
- Version 2.1: Release Date 16-Jan-17
- Added distribution graphs to most spreadsheets
- Version 2.0: Release Date 11-Jan-17
- TPD HIghly Variable Drug BE limit calculation added
- Version 1.9:
- Version 1.8:
- Fixed precision of ISV and ratio for BE sheets
- Updated to .xlsm format
- Version 1.7:
- Version 1.6:
- Alternate approaches for expansion of BE limits also included:
- Version 1.5:
- 'Calculate Power' button added to Bioequivalence worksheets
- Version 1.4:
- Now compatible with Excel 2007
- No extra library to install
- Opt-out warning at 1001 subjects
- Bioequivalence warning if expected ratio isn't within BE limits
- FARTSSIE calculates sample sizes for the following clinical trial designs:
- Superiority Trials: Parallel and 2-way Crossover
- Clinical Equivalence Trials: Parallel and 2-way Crossover
- Non-Inferiority Trials: Parallel and 2-way Crossover
- Bioequivalence Trials: Crossover, Replicate, and Parallel
- Trials to a Given Precision: Parallel and Crossover
- Difference Between Proportions (for smaller trials, up to 170 subjects)
- Variance Correction (baseline correction, post-dose measures correction, or both)
- A 'boss button' on most trial designs which will work backwards from the sample size your boss (or client) wants.
- No annoying advertisements, registration options, or guarantees.
Nquery software, free download. software
FeedbackI hope you find this tool useful. Did it work? Is there something you liked or disliked about it? Do you have a suggestion that could make it better? Your feedback is appreciated.
Email Me
Last Updated: 13-Oct-20